Innovation, Expetise and Diligence
Anchesen®
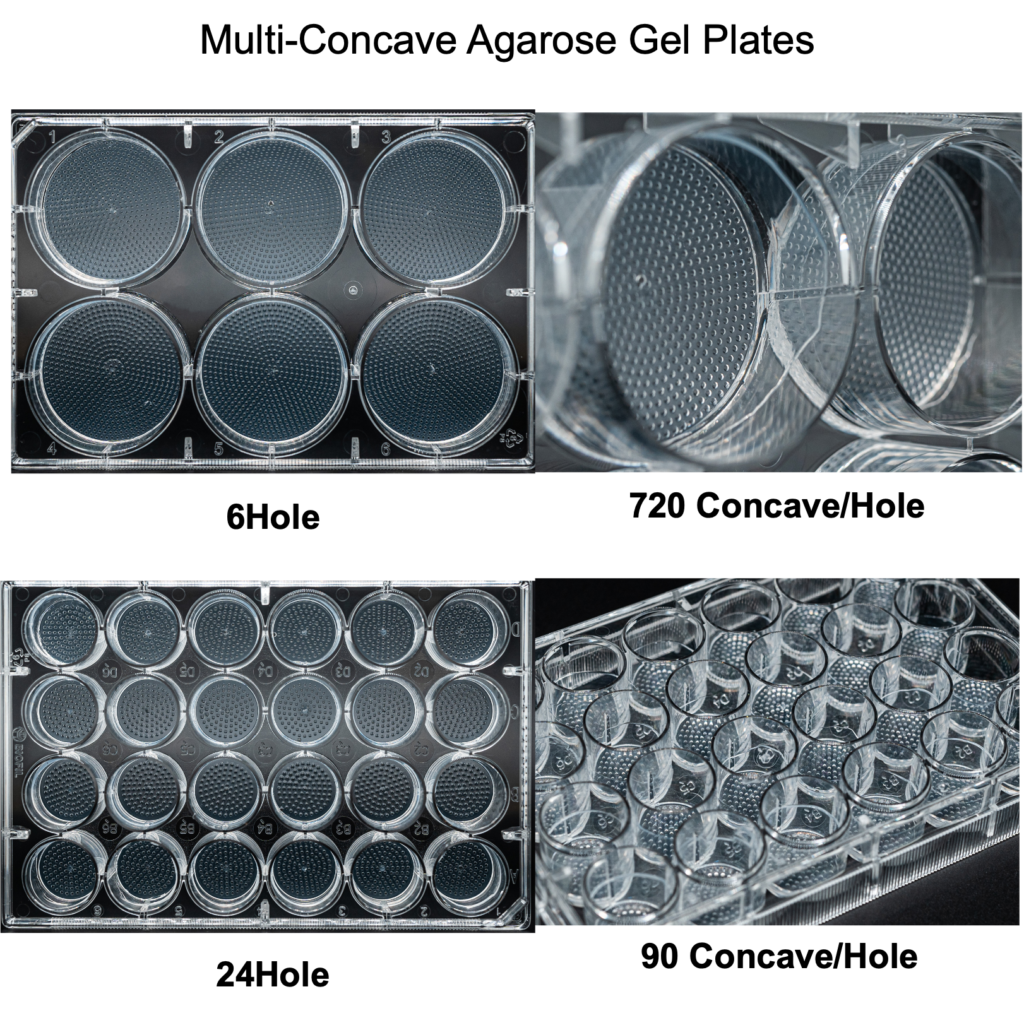
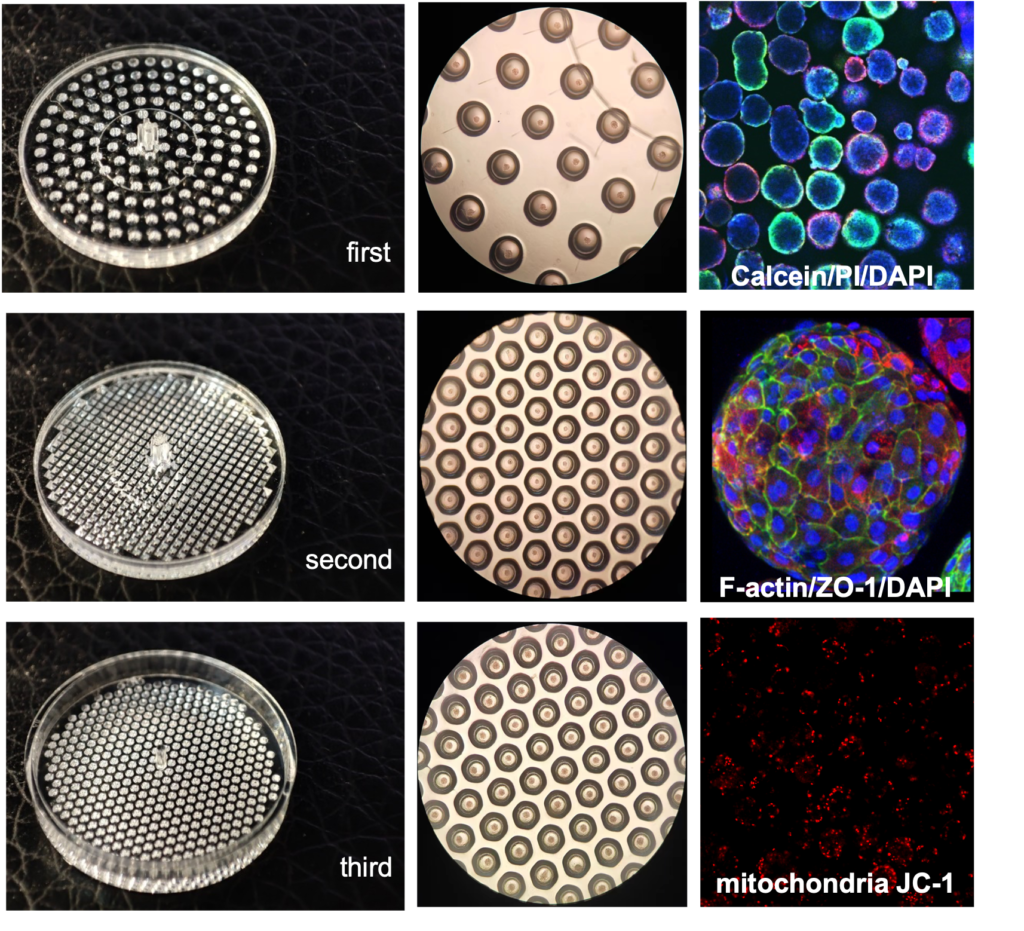
3D spheroid culture series
For several years, We have cultivated strong partnerships with Suzhou University and multiple life science companies, dedicated to enhancing the quality of cell culture research. Our commitment extends to the development of both 2D and 3D cell culture dishes. Technical features of our molds:
8-cavity mold with Full hot runner system
automated packaging,
cycle time <5.5 seconds,
daily production capacity >60,000 sets
Flat mirror surface grinding technology, reducing the warping deformation that can occur with manual polishing;
Due to the different weights of the upper and lower covers, Moldflow software is used for simulation analysis, controlling different gate diameters to achieve over 98% filling balance;
Modular design, facilitating replacement during production, reducing production maintenance costs and downtime losses.
CDOM Platform
Drug Hepatotoxicity Testing
In Vitro Metabolism DDI Testing
Long-Term Drug Metabolism Analysis
Drug Target Validation
Liver Disease Models
Small Nucleic Acid Drug Research
Multi-Organ Microfluidic Chips
Silicone-free Pre-filled Syringe from COC & COP for the most stable storage
Silicone oil has long been a staple in syringe manufacturing, serving as a crucial lubricant to mitigate friction and ensure the seamless, consistent movement of the plunger. This use of silicone oil has been necessitated by the challenges posed by the inherent properties of plastic materials.
The manufacturing process typically requires a draft angle to facilitate the release of the plastic item from the mold, which, in turn, makes it difficult to create syringe barrels with a zero-degree draft angle from plastics.However, the landscape is evolving, thanks to an innovation by Vivoid.
We’ve pioneered a groundbreaking injection mold technology with hot runner system capable of producing syringe barrels made from COC and COP materials with a revolutionary zero-degree draft angle, all while upholding the highest quality standards.
This remarkable achievement sets a new industry benchmark on a global scale.Silicone-free syringe barrels made from COC and COP materials offer an array of compelling advantages. First and foremost, they drastically lower the risk of contamination, ensuring the purity and integrity of the drugs or samples being handled. Their exceptional biocompatibility is a boon, assuring that these syringes are ideal for medical and pharmaceutical applications.
Second, they boast a lower risk of breakage, reducing potential disruptions in critical processes.
These syringe barrels exhibit minimal extractables and leachables, translating into a further layer of security for sensitive substances. Their high clarity provides visibility, a critical feature when precise measurements and observations are essential. Their robust chemical resistance allows them to withstand contact with a wide range of substances, making them versatile for various applications.
Furthermore, the low adsorption properties of COC and COP materials ensure that minimal interaction occurs between the material and the contents of the syringe, a vital attribute for maintaining sample integrity. Precision and reduced friction enhance user experience and contribute to consistent, accurate results. These materials also exhibit impressive heat resistance, expanding their utility in scenarios involving temperature variations.
Additionally, customization options cater to specific needs and preferences, further solidifying the status of silicone-free syringe barrels made from COC and COP materials as a preferred choice for applications where the maintenance of sample purity and integrity is of paramount importance. This innovation represents a significant leap forward in the field, offering heightened reliability and performance in critical pharmaceutical and medical processes.
